
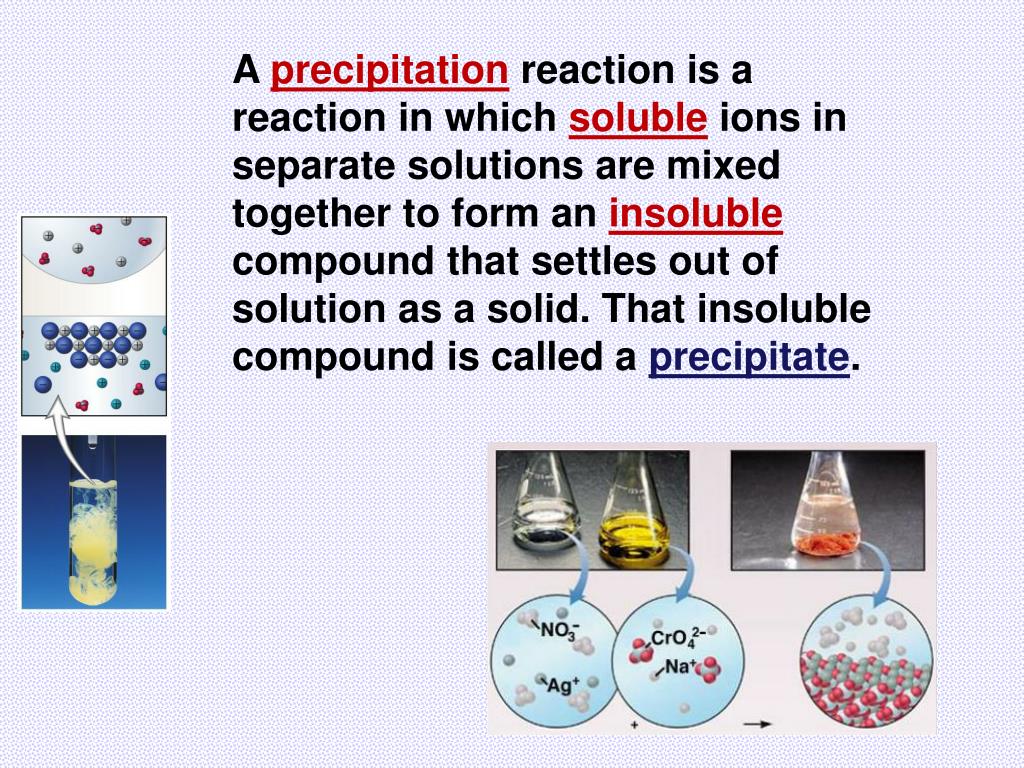
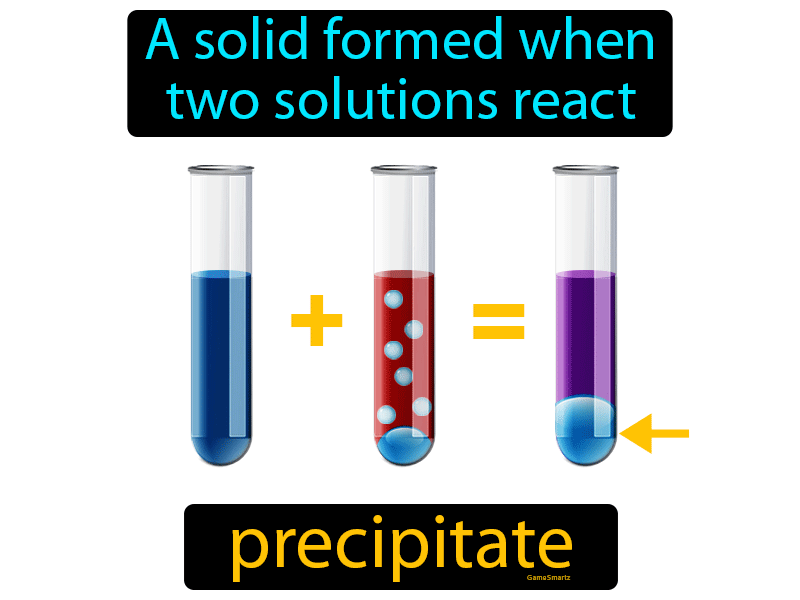
When a barium chloride solution reacts with sulphuric acid, a white precipitate of barium sulfate is formed. The formation of a precipitate can be caused by a chemical reaction. Precipitation occurs more rapidly from a strongly supersaturated solution. This can be due to temperature changes, solvent evaporation, or by mixing solvents. The precipitation of a compound may occur when its concentration exceeds its solubility. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases ( e.g., metallurgy and alloys) when solid impurities segregate from a solid phase.

The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the ' supernate' or ' supernatant'. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant. The solid formed is called the precipitate. In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. Similarly, barium ions are precipitated by sulfate ions, and calcium by oxalate schemes have been developed for analysis of mixtures of metal ions by successive application of reagents that precipitate specific ions or groups of related ions ( see qualitative chemical analysis).Principle of chemical precipitation in aqueous solution Precipitation often is used to remove metal ions from aqueous solutions: silver ions present in a solution of a soluble salt, such as silver nitrate, are precipitated by addition of chloride ions, provided, for example, by a solution of sodium chloride the chloride ions and the silver ions combine to form silver chloride, a compound that is not soluble in water. The distinction between precipitation and crystallization lies largely in whether emphasis is placed on the process by which the solubility is reduced or on that by which the structure of the solid substance becomes organized. SpaceNext50 Britannica presents SpaceNext50, From the race to the Moon to space stewardship, we explore a wide range of subjects that feed our curiosity about space!Ĭhemical precipitation, formation of a separable solid substance from a solution, either by converting the substance into an insoluble form or by changing the composition of the solvent to diminish the solubility of the substance in it.Learn about the major environmental problems facing our planet and what can be done about them! Saving Earth Britannica Presents Earth’s To-Do List for the 21st Century.100 Women Britannica celebrates the centennial of the Nineteenth Amendment, highlighting suffragists and history-making politicians.
PRECIPITATE CHEMISTRY HOW TO

Demystified Videos In Demystified, Britannica has all the answers to your burning questions.
Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives.Britannica Explains In these videos, Britannica explains a variety of topics and answers frequently asked questions.


 0 kommentar(er)
0 kommentar(er)
